what is necessary for a molecule to be polar
Polar and Non-Polar Molecules
Oil and h2o don't mix, right? That's why yous shake up your salad dressing; to temporarily force them together. Why does adding a piddling dish detergent (soap) help to remove the grease from muddy dishes better than water lone? Why does dry out cleaning lift stains that lather and water won't? The answer has to do with the chemical properties of the solvents we use, and the chemic properties of the things we are trying to dissolve (the solutes). We'll come back to these examples later.
oil and water don't mix
Chemical Bonds: Atoms seek more than stable states. The structure of an cantlet is similar to that of the solar organisation. The large protons (with a positive charge) and neutrons (with no accuse) are plant at the nucleus or center. The tiny electrons (with negative charges) circle chop-chop in orbits around the nucleus, forming electron shells at different distances, much like the planets and other objects that circumvolve the sun. Atoms of each element accept varying numbers of electrons in their outermost shells. Atoms go more stable when their outermost electron shells are emptied out or filled up. One way they tin achieve this goal is for 2 atoms to share one or more than electrons between them so that each of them can make full or empty that outermost shell. Only they tin only share the electron(s) if they stay close to each other, and this is chosen a covalent bond. In other situations, one cantlet can become more stable by losing electrons and the other can go more stable past gaining them. The atom that gained an electron (remember that electrons take a negative charge) becomes negatively charged (-ane) while the atom that lost an electron becomes positively charged (+1). Here's a little joke to assist you remember...

When an atom loses an electron, its internet charge goes from 0 (neutral) to +1 (positive)
The germination of an ionic bond is a redox reaction. I atom loses electrons (oxidation) while the other one gains electrons (reduction). Atoms that carry a accuse, either positive or negative, are called ions and, because opposites attract, they can grade an ionic bond. Ionic and covalent bonds are the about of import in all of chemical science. Here's a little joke to help you retrieve...

With ionic bonds, atoms give or accept electrons. With covalent bonds, they accept to share them.


the contrary poles of a magnet attract
Now call up virtually a magnet. Magnets take both a positive (+) pole and a negative (-) pole. So exercise batteries. So does the Earth. When things are different at each end, nosotros call them polar. Some molecules have positive and negative ends likewise, and when they do, we telephone call them polar. If they don't, we call them non-polar. Things that are polar can attract and repel each other (opposite charges concenter, alike charges repel). The ii magnets in the paradigm above volition attract because their opposite poles are near. Reverse one of them and they volition repel each other.
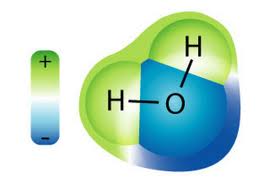
h2o molecules are polar
Water is a polar molecule. While the overall charge of the molecule is neutral, the orientation of the two positively charged hydrogens (+1 each) at ane terminate and the negatively charged oxygen (-two) at the other terminate give it two poles. This holding causes h2o molecules to be weakly attracted to other water molecules (positive to negative, negative to positive) and results in the cohesion of water to itself. Water droplets on a apartment piece of glass, for example, form droplets and bead upward.
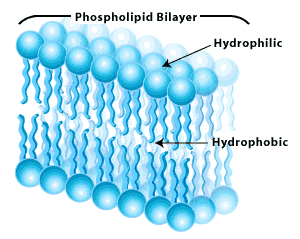
phospholipid bilayer of the cell membrane
Your cell membranes are made of phospholipid bi-layers. The polar heads (the roundphospho parts) confront the outsides and the non-polar tails (the lipids--remember that lipids are fats) face up the middle of the membrane. H2o, which is polar, therefore sticks to itself and it sticks to the phosphates on the outside and the inside surfaces of the membrane, just it is repelled (just the same way that oil and water repel each other) from the center of the membrane. The heads are hydrophilic (water loving) and the tails are hydrophobic (water fearing). This clever design makes cell membranes moist on their surfaces but watertight in the eye. Small non-polar molecules like oxygen and carbon dioxide can drift right through the membrane but annihilation polar or large is going to be stuck, and will need to exist actively transported through one of the prison cell's gates.

cross-section through a soap "micelle"
So why do soaps and detergents clean our dishes and our apparel? Soaps are chemically like to cell membranes. When soap is added to water, it forms structures called "micelles." The heads of the soap micelles are polar and the tails, which face inward to retreat from the polar water, are non-polar. When a soap micelle encounters oil or grease, these non-polar materials are forced to the within of the micelle to get away from the polar water and polar heads of the micelle, where they are trapped. When the soapy water is rinsed away, the trapped grease and oil is washed away with it.
Mini-Experiment ane: Pour some water into a shallow bowl. Now accept a length of thread or a long pilus and lay it on acme of the water in a closed loop. Put a few drops of vegetable oil inside the loop of thread and gently stir the oil. At present add some dish detergent outside the loop of cord and gently stir it into the h2o. Remove the thread and sentinel what happens.
Mini-Experiment 2: Hither's a dramatic experiment you can practise with food coloring, dish soap, and milk. Watch the video to see how information technology will look. Why does this work? Hint: milk contains fats, and lather repels fats. The food coloring is carried along in the milk as it retreats from the fats.
mclaughlinsplaccut.blogspot.com
Source: https://www2.nau.edu/lrm22/lessons/polar_nonpolar/polar_nonpolar.html
0 Response to "what is necessary for a molecule to be polar"
Postar um comentário